2 vapor appears as mixture comes to boil. The steps of the process are.

What Is Fractional Distillation Definition Process Video Lesson Transcript Study Com
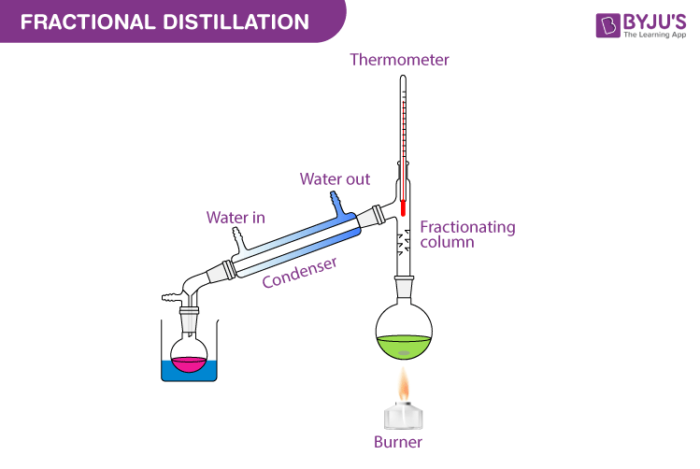
A simple distillation apparatus consists essentially of three parts.
. Under reduced pressure 2. A detailed edit history is available upon request. Automation Controls Engineering.
When the substance with the lowest boiling point has been removed the temperature can be raised and the distillation process repeated with the substance having the next lowest boiling point. Fractional distillation is useful for separating a mixture of substances with narrow differences in boiling points and is the most important step in the refining process. 5 Steps of Fractional Distillation.
On the right you can see several chemical processors that are described in the next section. Up to 24 cash back i Write a number 2 3 4 or 5 next to each stage so that the description of fractional distillation is in the correct order. A tall fractionating column is fitted above the mixture with several condensers coming off at.
Simple distillation i. Distillation refers to the process of using heating and cooling to separate and purify the components of a liquid mixture. 3 - after vaporization components begin to separate.
2 - vapor appears as mixture comes to boil. Crude Oil is heated to a high and temperature and vaporised and put into a column at the bottom. Distillation Process Contrary to popular belief distilling alcohol and making alcohol are two completely different processes.
4 as it rises vapor eventually cools. A condenser that consists of two tubes of different diameters placed one within the other and so arranged that the smaller in which the vapor is condensed is held in a stream of coolant in the. Concept of Process System Design.
Step-by-Step Procedures for Fractional Distillation is shared under a CC BY-NC-ND 40 license and was authored remixed andor curated by Lisa Nichols via source content that was edited to conform to the style and standards of the LibreTexts platform. Naturally to boil a substance its essential to heat it. The Fractional Distillation Process.
Preset reducing valve - is used only to give out the amount of pressure set by manifold. Some of the fractional distillation apparatuses in use for the process are distilling flask receiver thermometer heat source condenser and fractionating column. Fractional distillation the second process.
408 describe how the industrial process of fractional distillation separates crude oil into fractions. 5 cool down causes condensation and liquid is separately collected. After we set up the apparatus the mixture of liquids A and B which are miscible are taken where A has greater volatility than B substance.
Simple distillation is the process of separating components of a mixture containing two miscible liquids that boil without decomposition and have sufficient difference in their boiling points. The hydrocarbons vaporise and the gas rises. The Procedure of Fractional Distillation.
410 know the trend in colour boiling point and viscosity of the main fractions. The process of obtaining portions or fractions in this way is one type of fractional distillation. The Basic Distillation Process.
When a fraction in the vapours cools to its boiling point the fraction condenses. A flask equipped with a thermometer and with an outlet tube from which the vapor is emitted. Explore the definition of the distillation process and how it works to.
Preset reducing valve adjustable reducing valve and multiple stage reducing valve. 409 know the names and uses of the main fractions obtained from crude oil. Boiling temperatures are what enable fractional distillation to be each relatively easy and affordable.
Crude oil vapour is put into a fractionating column at the bottom and rises upwards. 1 beer feed rate and temperature 2 column pressure and temperature for both the stripping and the rectifying sections 3 reflux- to-product flow rate to control the column discharge temperature and alcohol vapor concentration and 4 heat. Remember that a continuously operating distillation process must simultaneously and continuously monitor and regulate.
1- heat mixture to begin boiling. In two or more complete sentences describe the five step process of fractional distillation that is used to refine crude oil. For example liquid ethanol can be separated from a mixture of ethanol and water by fractional.
Commonly used with Bourdon gauges. Even substances which have extremely comparable boiling temperatures can be accurately separated by fractional distillation. Fractional distillation separates hydrocarbons using their different boiling points.
Numbers 1 and 6 have been done for you. This method is used to separate a mixture of solid into a liquid. Crude oil is heated until it evaporates.
This gives off heat and is the only source of heat- meaning there is a hot bottom and cool top. Fractional distillation is a method for separating a liquid from a mixture of two or more liquids. Fractional distillation separates a mixture into a number of different parts called fractions.
Adjustable reducing valve - is used on the high pressure system to the regulator from high to low pressure to the system. 5 Steps of Fractional Distillation. All alcohol be it in beer wine whiskey or vodka is not even produced by a brewer winemaker or distiller - it is produced.
Fractional distillation- the first process. 3 after vaporization components begin to separate. 2 Number Stage 1 The crude oil is heated to 350 C.
The oil refining process starts with a fractional distillation column. 1- heat mixture to begin boiling. Under atmospheric pressure ii.
Refinery gases gasoline kerosene diesel fuel oil and bitumen. Theoretical consideration Basic Concepts Vapour Pressure According to Kinetic Theory the molecules in a liquid are in a constant state of thermal motion and some of these molecules are moving fast enough to.

What Is Fractional Distillation Definition Process Video Lesson Transcript Study Com

Basic Steps To Fractional Distillation Understanding Distillation

Fractional Distillation Detailed Explanation Along With Diagrams
0 Comments